Within mixed microbial communities, the various bacterial members constantly interact with each other, both cooperatively and competitively. Aggressive competition between bacteria can determine which species prosper in a community and is often mediated by delivery of antibacterial protein toxins from one bacterium to another1. Writing in Nature, Zhao et al.2 describe a previously unknown class of self-delivering antibacterial toxins that form a distinctive umbrella-like structure and that might be used by many bacteria in soil and other ecosystems to block the growth of their competitors. This discovery expands the portfolio of mechanisms by which bacteria kill or disable their rivals and offers exciting potential for future exploitation.
Read the paper: Streptomyces umbrella toxin particles block hyphal growth of competing species
Bacteria can use a variety of approaches to harm their competitors. These include the production of diffusible antibiotics and protein toxins, and the direct injection of toxins into neighbouring cells in a contact-dependent manner1. Competition is intense in environments in which the microbial community is dense and complex, such as soil and the human gut, and is often fiercest between related bacteria that have similar needs3.
Many protein toxins involved in interbacterial competition are known as polymorphic toxins, to reflect the existence of multiple versions of the same protein. Polymorphic toxins are large modular proteins that contain varied, exchangeable toxin domains joined to common domains that are required for delivery of the toxin domain from the producer into the recipient ‘target’ bacterium4.
Interestingly, many of these toxin domains are found in different polymorphic toxins with unrelated delivery mechanisms. Building on earlier work that used this observation to generate a large data set of predicted polymorphic toxins5, Zhao and colleagues selected candidate toxins to investigate and chose examples that are found in the ubiquitous soil bacteria Streptomyces. These were of particular interest because although Streptomyces exist in a competitive soil environment and produce numerous small antimicrobial molecules6, they had not been previously reported to use polymorphic protein toxins for interbacterial competition.
The authors studied three of the candidate toxins, UmbC1, UmbC2 and UmbC3, in the model bacterium Streptomyces coelicolor. Each UmbC protein has a predicted toxin domain at one end and interacts with two other proteins — a specific UmbA and UmbB protein encoded in the same set of genes — to form a protein complex. Three additional UmbA proteins can associate with any of the UmbC-containing assemblies. Structural analysis revealed that Umb complexes form large particles (Fig. 1) with an unusual umbrella-like shape that has not been reported before — hence the name umbrella toxins.
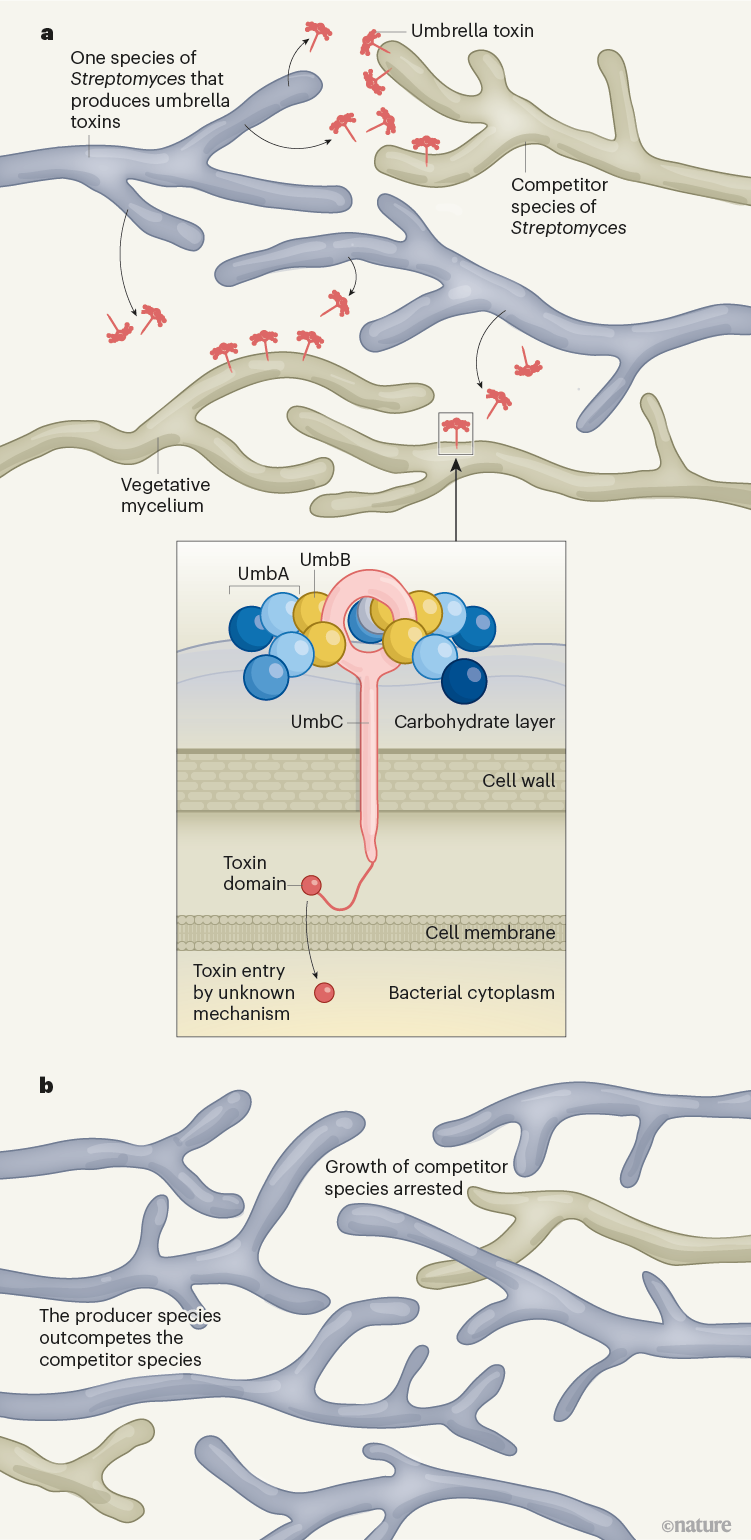
Figure 1 | Streptomyces bacteria produce umbrella toxins to inhibit the growth of their competitors. Streptomyces are soil-dwelling bacteria that occupy dense microbial communities and grow in networks of branching filaments called a vegetative mycelium. Zhao et al.2 report that Streptomyces and related bacteria produce a previously unknown class of antibacterial toxin, called an umbrella toxin. a, One species of Streptomyces (grey) can target another species of Streptomyces (beige) by releasing a version of these antibacterial agents. Umbrella toxins contain a central lollipop-shaped UmbC protein, which carries the toxin domain, and five ‘spokes’ formed by UmbB and UmbA proteins. Several UmbA proteins that contain domains called lectin domains are incorporated in the same umbrella toxin (different blue shading indicates different lectin domains). These lectin domains probably enable the umbrella toxin to dock onto susceptible bacteria by binding to specific cell-surface carbohydrates. The antibacterial toxin domain of UmbC varies between umbrella toxins. The stalk of UmbC might protrude through the outer layers of the cell to deliver the toxin domain to the cell wall or membrane. How the toxin crosses the cell membrane to act inside the cell is currently unclear. b, The umbrella toxin inhibits growth of the competitor species.
In each toxin-containing particle, UmbC forms a lollipop shape, comprising a ring joined to a long stalk with the toxin domain at the end of the stalk away from the ring. Five ‘spokes’ extend out from the ring, each containing UmbB and then UmbA, although it was not possible for the authors to determine which of the four possible UmbA proteins was present in which spoke or spokes. Interestingly, each UmbA protein contains a different predicted lectin domain that forms the exposed tip of the spoke. Given that functional lectin domains bind to specific carbohydrates, it is probable that interaction between the UmbA lectin domain and carbohydrate on the surface of target cells enables the umbrella toxin to dock onto and recognize target cells that display the required carbohydrate.
Umb particles secreted by S. coelicolor were collected by Zhao and colleagues and administered to a range of bacterial species. The particles inhibited growth, but only for certain species of Streptomyces and other bacteria in the same grouping (Actinobacteria) as that of Streptomyces. This evidence indicates that Umb toxins do not need to be injected by the producing cell to cause harm to recipient cells, but can reach susceptible bacteria once they are released into the external environment. Further examination revealed that the toxin particles studied inhibit a particular part of the Streptomyces life cycle — growth in a branching filamentous form known as a vegetative mycelium.
The three UmbC toxin domains were each confirmed to have antibacterial activity. One modifies DNA and two are previously unknown toxins that are predicted to compromise the cell wall or the membrane of targeted cells. The authors identified genes encoding umbrella toxins in 875 species of Actinobacteria, which suggests that Umb-mediated competition in this group (which includes soil bacteria and bacteria that cause human disease6) is widespread. The potential diversity of these toxins is striking, with 77 distinct toxin domains for UmbC proteins and at least 20 distinct lectin domains for UmbA proteins.
The discovery of any previously unknown class of antibacterial toxin-delivery system is exciting because it expands our knowledge of how to incapacitate bacterial cells and how competitive interactions contribute to microbial ecology. Umbrella toxins are particularly interesting because of a number of unique features, including the distinctive structure of the Umb particles, the probable use of specific carbohydrates on target cells for toxin docking, and the ability of the toxins to mediate competition between Actinobacteria.
The journey to understand previously unknown microbial genes
Looking to future experiments, the idea that UmbA lectins mediate species-specific docking of Umb particles remains to be proved, and the molecules that are recognized on target-cell surfaces still need to be identified. Perhaps the incorporation of different UmbA proteins that recognize different surface motifs into all or selected Umb particles will increase the number of species that are susceptible, or prevent resistance to the toxin if target cells lose one motif. Once the particle is docked, it is unknown how the toxin domains reach their ultimate destination inside the targeted cells.
A final intriguing question is whether Umb particles are always antibacterial toxins. Most sets of umb genes also encode a predicted immunity protein, which would protect the producer against its own UmbC toxin, but some sets do not encode such a protein. This lack of an immunity protein might simply reflect a failure to identify the gene, but might imply that some Umb particles have non-bacterial targets (such as animals, plants or fungi) or are used for interbacterial communication rather than for causing harm.
Umbrella toxins could potentially be developed for use as tailored antimicrobials — human or plant pathogens could be treated with purified Umb particles or Umb-secreting ‘biocontrol’ bacteria. It might even be possible to engineer designer Umb toxins to recognize and kill particular pathogens by incorporating specific lectin domains in the constituent UmbA proteins and a suitable toxin in UmbC. This would be similar to the approach of engineering secreted bacterial ‘syringes’ into designer protein-delivery vehicles7. More broadly, the discovery of umbrella toxins supports the idea that there are more antibacterial toxin systems to be found. Such discoveries will in turn create further opportunities to develop antimicrobial strategies and offer new insights into how microorganisms compete.

 Read the paper: Streptomyces umbrella toxin particles block hyphal growth of competing species
Read the paper: Streptomyces umbrella toxin particles block hyphal growth of competing species
 The journey to understand previously unknown microbial genes
The journey to understand previously unknown microbial genes
 The language of bacterial defences expands
The language of bacterial defences expands